At the end of July the annual AVS International Conference on Atomic Layer Deposition (ALD) – combined with the Atomic Layer Etching (ALE) Workshop – took place in Bellevue, Seattle. As customary, the Sunday before the conference itself was devoted to tutorials, each about 45 minutes long. In previous years the focus was mainly on fundamentals of ALD and ALE. This year, the organizers chose to include also applications, with selected topics being area-selective ALD, atomic layer etching, catalysis, batteries and solar cells.
I was very happy to be invited to give a tutorial on ALD for Photovoltaics, i.e. solar cells. However, I quickly realized it would be quite a challenge to give a 45-minute overview of the very diverse field of ALD for Photovoltaics, to an audience which is mostly ALD-oriented (~800 people by the way!). Therefore, I chose to focus on those applications where certain merits of ALD (e.g. conformality, thickness control, ….) really make the difference. The four take-away messages I selected are the following:
I then first gave a crash course on solar cells and organised the applications of ALD into three topics:
- ALD4SiliconPV: ALD for passivation layers and passivating contacts
- ALD4TCOs: ALD for transparent conductive oxides (TCOs)
- ALD4Perovskite: ALD in the upcoming field of perovskites and tandem cells
My intention is certainly not to repeat all of this here! In case you are interested in the slides, feel free to download them here:
Instead, what I rather want to do in this blogpost is to connect to a question from the audience during the Q&A after my tutorial. It went something like ….
“You presented many examples of where you can use ALD in solar cells. Which concepts are really being used now in industry and which ones do you expect to play a role in the future?”
My intention for this blog is to write down (in more detail) the I answer to this question. Therefore, I will first show where ALD is used in solar cells you can buy today, followed by applications where I personally see opportunities for ALD to be used in future solar cells.
ALD is already big in the solar industry!
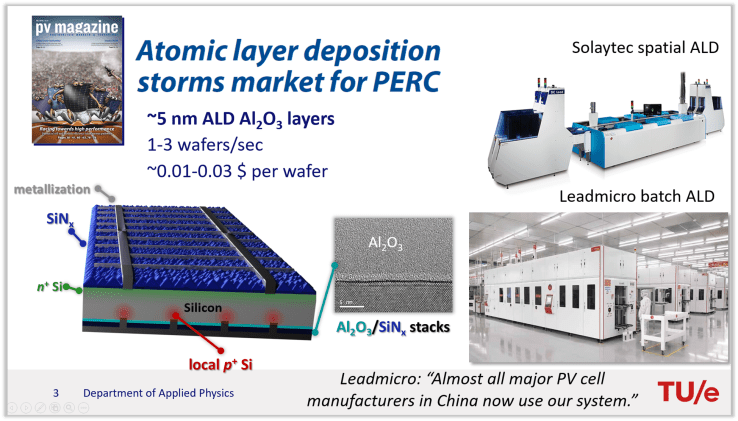
I started my tutorial on a positive note, where I wanted to convey the message that ALD is already really big in the solar industry. I gratefully used some quotes from a recent article in PV Magazine , “Atomic Layer Deposition storms market for PERC”. Quite a pompous title!
The 3D solar cell scheme I rendered on the bottom left of the slide above shows a silicon wafer-based solar cell type called PERC (Passivated Emitter and Rear Contact). PERC is now rapidly becoming the dominant solar cell technology and will be in the foreseeable future according to the ITRPV roadmap. Without explaining the full solar cell, one of the key elements of such a PERC cell is the teal layer I drew near the bottom, which is a ~ 5nm thick Al2O3 layer. This Al2O3 layer enables efficiency gains of over 1% absolute! There are more details in the slides, but in a nutshell: The Al2O3 works so well since it provides excellent passivation of defects at the rear silicon surface. Although there are more materials that can passivate defects (SiO2, SiNx, ….) , the unique feature of Al2O3 (especially at the time of discovery) was that it exhibits a negative fixed charge. This negative charge repels holes from the silicon surface, which makes it a great passivation material for p-type silicon, which is the absorber material in PERC. Together with IMEC, our Plasma & Materials Processing group at the Eindhoven University of Technology was a pioneer in demonstrating and understanding the potential of ALD Al2O3 in PERC cells, and it is great to see that this ALD application has really made it to market.[1]–[3] For those who want to learn more about passivation and Al2O3, I’d recommend the following articles and refer back to our previous blog post The prospects for the use of Al2O3 in solar cells (in 10 questions) – How we looked at it in 2011 . [4]–[7]
Within the market for Al2O3 deposition in PERC cells both PECVD and ALD tools are available, where ALD tools come both in spatial and batch ALD tool flavours. Both PECVD and ALD approaches have their strong points.For example, ALD tends to yield better film quality – especially for ultrathin films – and has better precursor consumption. PECVD tools on the other hand can easily do single-sided deposition and can also be used for the subsequent SiNx deposition step, which also reduces the amount of wafer handling steps. There is also interest in doing (ALD or PECVD) Al2O3 in tube PECVD SiNx systems, which also saves handling. Among others, Tempress and Centrotherm offer this solution. A nice comparison on the available industrial ALD and PECVD tools in terms of throughput, uptime, film thickness and TMA consumption can be found in this 2018 Taiyang report.
When considering the market shares of PECVD and ALD, it seems that the field is changing quite rapidly. As the 2018 Taiyang report mentions, PECVD supplier Meyer Burger was at the time “undoubtedly the market leader” with an estimated market share of 60-70%. However, especially the company LeadMicro seems to be gaining a substantial market share with their batch ALD Al2O3 concept. Two quotes from LeadMicro in the recent PVMagazine article nicely show the scale:
“We have so far equipped over 30 GW of manufacturing lines with ALD passivation systems …”
I was personally surprised by the 30 Gigawatt (GW) number, which is really a lot in a total market of ~100-120 GW. Also, since one wafer gives about 5-6 W of solar power, a quick calculation shows that a staggering figure of about 5-6 billion wafers are coated by ALD Al2O3 annually!
“Leadmicro plans on revealing the latest ALD system in its Kuafu line of products designed to passivate 10,000 wafers an hour.”
This quote I think very nicely shows that – contrary to popular opinion – ALD is not inherently slow! 10,000 wafers an hour means about 3 wafers per second! I personally think that this throughput is really enabled by the fact that the ALD Al2O3 process is a beautiful process: Trimethylaluminum (TMA) has a very high reactivity and very good saturation behaviour. In addition, TMA is relatively cheap! For solar cells, you can only spend a few cents on a layer like Al2O3, quite a contrast to e.g. the application of ALD in semiconductor processing!
By the way, I am curious to see how things will evolve from here in this PECVD vs ALD “race”. The opinions seem to vary! In this article in the July edition of PV Magazine you can read about the opinions of various academic and industrial entities.
Where I see potential for ALD to be used:
-
ALD Al2O3 for hydrogenation of poly-Si passivating contacts
The more “near-future” concept where I see ALD play a potential role is in so-called poly-Si passivating contacts, shown in the slide below.
What you see here is a high efficiency “TOPCon” silicon solar cell. First of all, it features a p+ front silicon surface, where ALD Al2O3 is again applied for surface passivation. What I would to focus on however is a new application for ALD Al2O3 at the rear side. The rear side features an ultrathin, ~1.5 nm, SiO2 layer (yellow) followed by an n-type doped poly-Si layer (green), which is a very good electron-collector (n-type contact). This is actually quite old technology from the transistor-world, and was more or less “rediscovered” for solar cells by Fraunhofer ISE back in 2013. As you can see from the top-right graph, these contacts have quickly enabled very efficient cells, and now in 2019 solar panel companies such as Trina Solar are actually launching such “TOPCon” modules! [8] The SiO2 and poly-Si are both however not made by ALD! So where could ALD be used here?
Actually one of the key steps to making efficient poly-Si contacts is hydrogenation of the SiO2 tunnel oxide. For those not familiar with this: In the SiO2 layer there are defects at the atomic level (e.g. unsaturated, dangling bonds) that can lead to loss of charge by recombination. By bringing hydrogen to the SiO2, i.e. hydrogenation, hydrogen can bond to these defects which then get passivated. This leads to less recombination loss and consequently to a higher cell voltage.
It turns out that ALD Al2O3 is an excellent source of hydrogenation for the SiO2 interface.[9] As shown schematically in the coloured TEM image on the slide, ALD Al2O3 contains typically a few atomic percent of hydrogen in the as-deposited state. This H can migrate to the SiO2 layer upon thermal annealing, typically at 400-450 oC. Not only does the Al2O3 provide hydrogen, it is also very good at keeping the H in, i.e. it is a dense capping layer, especially when combined with a standard SiNx anti-reflection coating. Keeping the hydrogen in is very important for subsequent processing of the solar cell, where for example the metal contacts are “fired” at temperatures typically well in excess of 750 oC.
Therefore, I can imagine that with the current introduction of ALD Al2O3 for PERC, there is also the potential to use ALD Al2O3 tools for these SiO2/poly-Si passivating contacts. Especially if you consider that ALD can be done on both sides of the wafer, there might be opportunities to apply Al2O3 simultaneously on one side of the wafer as a hydrogenation and capping layer, and on the other side as conventional passivation layer. I wouldn’t be surprised if companies are already doing this!
Finally, I think there is increasing interest in adding a TCO on top of the poly-Si.[10], [11] This would help in making a bifacial solar cell (light can enter cell from both sides) or can help in avoiding (fired) metallization-induced damage to the poly-Si. Since ALD TCOs are often rich in H (and don’t induce plasma damage, as I will elaborate below) , I see potential for ALD TCOs such as ZnO rather than Al2O3 to serve as hydrogenation source for the SiO2 tunnel oxide.[10], [12], [13]
-
ALD for hybrid metal halide perovskite and Si-perovskite tandems
So far I’ve been talking about solar cells based on silicon wafers, where the silicon is the light-absorbing material. Although this is and will remain to be the dominant technology in the foreseeable future, there is a strong academic – and increasingly industrial – interest in so-called hybrid organic/inorganic metal halide perovskite solar cells. As you can see in the image below, this relatively recently-discovered class of solar cells has made great strides in efficiency in a matter of a few years. Besides purely perovskites, there’s also great interest in silicon-perovskite tandem cells, where you place a perovskite solar cell on top of a silicon solar cell. The perovskite top cell is more sensitive to high-energy blue light, whereas the silicon bottom is more sensitive to low-energy red light. Combined, these cells have been shown to reach 28% conversion efficiency, which is already higher than the 26.7% record silicon solar cell and close to the absolute 29.4% limit for a silicon cell alone.[14] In addition, the companies OxfordPV and Meyer Burger will start pilot production of silicon-perovskite tandem cells already at the end of next year! Also worth mentioning are CIGS-perovskite tandems which can potentially provide flexible, light-weight and efficient tandems.
The role for ALD in the field of perovskites and tandems mostly in preparing the charge extraction/transport layers and engineering the interfaces. A prime example here is ALD SnO2, which is used in pretty much all the recent record Si-perovskite tandem cells.[15]–[17] You can see the configuration of the (many!) layers in such a Si-perovskite tandem cell in the image I rendered below.

Note that this image is certainly not to scale (the silicon wafer is typically 160 µm thick, while the perovskite absorber is only a few hundred nm).
Typically, ALD SnO2 is deposited on top of C60 or PCBM which is the electron contact to the perovskite. On top of the ALD SnO2, an indium-zinc-oxide (IZO) TCO is sputtered. Seeing this, you might wonder: Why not directly sputter the TCO on top of the C60? The reason is quite simple: Without the typically 10-20 nm ALD SnO2 as a protective buffer layer, the perovskite would not survive the harsh plasma conditions during sputter deposition of the IZO TCO. Plasma radiation (VUV photons) and ion bombardment are notorious for inducing defects in solar cell structures.[18]–[21] As opposed to sputtering, the absence of plasma in thermal ALD makes it clearly a soft deposition process. Therefore, ALD allows to deposit a protective buffer layer without damaging the perovskite. Since I personally do not see an easy industrial alternative to sputter deposition of the thick top TCO, I think ALD SnO2 will keep on playing a key role in these tandem structures. Note also that ALD companies like SoLayTec are already advertising on having spatial ALD SnO2 processes available for perovskites.
As I stressed in my tutorial, I see this concept of soft ALD deposition becoming increasingly important for novel cell concepts. See the slide below:
What I show (from left to right) are solar cell contacts and concepts which increasingly suffer from plasma damage. The surface passivation of amorphous silicon (a-Si:H) layers, often found in silicon heterojunction solar cells, in the left image degrade under plasma. However, this damage can almost be fully recovered by thermal annealing.[18], [22], [23] For the SiO2/poly-Si passivating contacts I already mentioned above, typically a non-perfect recovery is observed by annealing.[10] Therefore, soft ALD deposition of TCOs can be an advantage here. For the 3rd and 4th examples, direct plasma exposure has to be avoided altogether: As mentioned above, perovskite cells need a protective buffer layer (e.g. ALD SnO2) before a material can be sputtered on top. Similarly, a harsh plasma has to be avoided when depositing on ultrathin (~1-2 nm) tunnel oxides, such as in the right image example of a passivating SiO2/ALD ZnO stack.[24] Since ultra-thin oxides are becoming ever more prominent in new passivating contact concepts, I also see a role for soft ALD deposition here.[6]
Coming back to the perovskites: One of the interesting applications of ALD would be to deposit ALD functional layers directly on top of the perovskite. However, even though thermal ALD does not induce plasma-damage, performing ALD directly on perovskites can be challenging.[25] Firstly, the limited thermal stability of perovskites limits the deposition temperature to typically 100-120 oC. Also, the precursor chemicals of certain ALD processes can interact with the perovskite! This is for example known for the thermal ALD SnO2 process employing TDMASn (Sn(NMe2)4) and H2O, used in the Si-perovskite tandem cells mentioned above. The ligands of the TDMASn precursor are known to extract the organic cations from the perovskite, especially at temperatures exceeding 120 oC. By keeping the deposition temperature sufficiently low and having C60 below the SnO2, excessive “chemical damage” by the ALD precursor can however be kept at bay.[26]
This does however not mean that one cannot do ALD directly on top of perovskite! A very nice example of what ALD can do for perovskites is shown on the slide below. ALD can be used to coat the perovskite with a conformal and ultrathin Al2O3 layer (using TMA and H2O) without excessively damaging the perovskite.[27] As can be seen in the middle graph, the addition of just a few cycles of ALD Al2O3 enhances the efficiency of the perovskite cell considerably, which is attributed to a passivation effect.[28] However, if the Al2O3 layer is too thick (>15 cycles, which is nominally ~1.6 nm Al2O3) the Al2O3 becomes too thick for tunnelling, leading to an insulating barrier and thus reduced cell efficiency. To my opinion, the ability of ALD to conformally coat the perovskite absorber (which can have quite a high surface roughness) with a precise control over the thickness at the ~0.1 nm level really enables reaching this gain! In addition to enhancing the cell efficiency, the Al2O3 layer also improves the stability of the (unencapsulated!) cell during damp-heat stability tests, as shown in the right graph.
Concluding remarks
To conclude, I think that the more we creep towards the solar cell efficiency limits, the more important the merits of ALD will become for further improvements. Here I made a short list of these merits and some examples of their use.
- Ångström-level thickness control
ALD can prepare ultrathin layers that can for instance passivate while still allowing for tunneling.[28] - Conformality
Being able to conformally coat high aspect ratio features enables the use of for instance black silicon or CIGS-perovskite tandems where ALD NiO conformally coats the rough CIGS bottom cell.[29] - Many ALD materials
As evidenced by our rapidly growing ALD process database, ALD can provide many new materials which can for instance function as novel passivation or passivating contact materials.[6] - Soft deposition
The absence of deposition damage becomes increasingly important for new ultrathin passivating (oxide) materials and perovskites. - Composition control
The film composition can be accurately tuned by ALD through the use of supercycles. For example, the doping level in TCOs can be easily varied or even be graded[5], which can allow for a more independent optimization of the optical losses and electrical contacting. - Pinhole-free layers
The low pinhole density of ultrathin ALD layers is increasingly important for environmental stability and preventing shunts. - Any others I forgot? Leave a comment! 🙂
Which of these emerging applications will make it to industry will probably be mostly a matter of cost considerations. Therefore, I think that the search for inexpensive and highly reactive precursor chemistries is of high importance for this field. Connected to that, I also think that the emergence of high-throughput spatial and batch ALD systems with excellent precursor utilisation is truly a feat of engineering that also proves that ALD is not inherently slow. So I wouldn’t be suprised if in a few years’ time the field of “ALD4PV” will have moved beyond “just Al2O3“. Exciting times!
AtomicLimits Image Library
As a final note on this blogpost: We are in progress of making an AtomicLimits image database. Here we will put images to be re-used by the community. Although this image database is work in progress, I have already put some of the 3D-rendered solar cell structures I used in my tutorial on the page. So feel free to grab them here!
References
[1] B. Hoex, S. B. S. Heil, E. Langereis, M. C. M. Van De Sanden, and W. M. M. Kessels, “Ultralow surface recombination of c-Si substrates passivated by plasma-assisted atomic layer deposited Al2O3,” Appl. Phys. Lett., vol. 89, no. 4, p. 042112, 2006.
[2] B. Hoex, J. Schmidt, P. Pohl, M. C. M. van de Sanden, and W. M. M. Kessels, “Silicon surface passivation by atomic layer deposited Al2O3,” J. Appl. Phys., vol. 104, no. 4, p. 044903, 2008.
[3] G. Agostinelli, A. Delabie, P. Vitanov, Z. Alexieva, H.F.W. Dekkers, S. De Wolf, G. Beaucarne, “Very low surface recombination velocities on p-type silicon wafers passivated with a dielectric with fixed negative charge,” Sol. Energy Mater. Sol. Cells, vol. 90, no. 18–19, pp. 3438–3443, Nov. 2006.
[4] G. Dingemans and W. M. M. Kessels, “Status and prospects of Al2O3-based surface passivation schemes for silicon solar cells,” J. Vac. Sci. Technol. A Vacuum, Surfaces, Film., vol. 30, no. 4, p. 040802, 2012.
[5] B. Macco, B. W. H. van de Loo, and W. M. M. Kessels, Atomic Layer Deposition for High Efficiency Crystalline Silicon Solar Cells. Wiley, 2017.
[6] L. E. Black, B. W. H. Van De Loo, B. Macco, J. Melskens, and W. M. M. Kessels, “Explorative studies of novel silicon surface passivation materials : Considerations and lessons learned,” Sol. Energy Mater. Sol. Cells, vol. 188, no. June, pp. 182–189, 2018.
[7] J. Melskens, B. W. H. van de Loo, B. Macco, L. E. Black, S. Smit, and W. M. M. Kessels, “Passivating Contacts for Crystalline Silicon Solar Cells: From Concepts and Materials to Prospects,” IEEE J. Photovoltaics, vol. 8, no. 2, pp. 373–388, Mar. 2018.
[8] Y. Chen et al., “Mass production of industrial tunnel oxide passivated contacts (i‐TOPCon) silicon solar cells with average efficiency over 23% and modules over 345 W,” Prog. Photovoltaics Res. Appl., no. July, p. pip.3180, Jul. 2019.
[9] M. Schnabel et al., “Hydrogen passivation of poly-Si/SiOx contacts for Si solar cells using Al2O3 studied with deuterium,” Appl. Phys. Lett., vol. 112, no. 20, 2018.
[10] L. Tutsch et al., “Implementing transparent conducting oxides by DC sputtering on ultrathin SiOx/ poly-Si passivating contacts,” Sol. Energy Mater. Sol. Cells, vol. 200, no. May, p. 109960, 2019.
[11] E. Bruhat, T. Desrues, D. Blanc-Pélissier, and B. Martel, “TCO contacts on poly-Si layers : High and low temperature approaches to maintain passivation and contact properties TCO Contacts on Poly-Si Layers : High and Low Temperature Approaches to Maintain Passivation and Contact Properties,” vol. 040001, no. August, 2019.
[12] B. Macco, H. C. M. Knoops, M. A. Verheijen, W. Beyer, M. Creatore, and W. M. M. Kessels, “Atomic layer deposition of high-mobility hydrogen-doped zinc oxide,” Sol. Energy Mater. Sol. Cells, vol. 173, pp. 111–119, Dec. 2017.
[13] B. Macco, H. C. M. Knoops, and W. M. M. Kessels, “Electron Scattering and Doping Mechanisms in Solid-Phase-Crystallized In2O3:H Prepared by Atomic Layer Deposition,” ACS Appl. Mater. Interfaces, vol. 7, no. 30, 2015.
[14] A. Richter, M. Hermle, and S. W. Glunz, “Reassessment of the limiting efficiency for crystalline silicon solar cells,” IEEE J. Photovoltaics, vol. 3, no. 4, pp. 1184–1191, 2013.
[15] K. A. Bush et al., “23.6%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cells with Improved Stability,” Nat. Energy (under Rev., no. February, pp. 1–7, 2017.
[16] M. Jošt et al., “Textured interfaces in monolithic perovskite/silicon tandem solar cells: Advanced light management for improved efficiency and energy yield,” Energy Environ. Sci., 2019.
[17] E. Köhnen et al., “Highly efficient monolithic perovskite silicon tandem solar cells: analyzing the influence of current mismatch on device performance,” Sustain. Energy Fuels, 2019.
[18] B. Demaurex, S. De Wolf, A. Descoeudres, Z. C. Holman, and C. Ballif, “Damage at hydrogenated amorphous/crystalline silicon interfaces by indium tin oxide overlayer sputtering,” Appl. Phys. Lett., vol. 101, no. 17, p. 171604, 2012.
[19] B. Demaurex et al., “Atomic-layer-deposited transparent electrodes for silicon heterojunction solar cells,” IEEE J. Photovoltaics, vol. 4, no. 6, 2014.
[20] H. B. Profijt, S. E. Potts, M. C. M. van de Sanden, and W. M. M. Kessels, “Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges,” J. Vac. Sci. Technol. A Vacuum, Surfaces, Film., vol. 29, no. 5, p. 050801, 2011.
[21] Y. Kuang et al., “Towards the implementation of atomic layer deposited In2O3:H in silicon heterojunction solar cells,” Sol. Energy Mater. Sol. Cells, vol. 163, no. January, pp. 43–50, 2017.
[22] B. Macco, D. Deligiannis, S. Smit, R. A. C. M. M. Van Swaaij, M. Zeman, and W. M. M. Kessels, “Influence of transparent conductive oxides on passivation of a-Si:H/c-Si heterojunctions as studied by atomic layer deposited Al-doped ZnO,” Semicond. Sci. Technol., vol. 29, no. 12, 2014.
[23] B. Demaurex et al., “Atomic-Layer-Deposited Transparent Electrodes for Silicon Heterojunction Solar Cells,” IEEE J. Photovoltaics, vol. 4, no. 6, pp. 1387–1396, Nov. 2014.
[24] B. W. H. van de Loo, B. Macco, J. Melskens, W. Beyer, and W. M. M. Kessels, “Silicon surface passivation by transparent conductive zinc oxide,” J. Appl. Phys., vol. 125, no. 10, p. 105305, 2019.
[25] V. Zardetto, F. Di Giacomo, M.A. Mohammed, G. Lucarelli, S. Razza, A. D’Epifanio, S. Licoccia, W.M.M. Kessels, A. Di Carlo, T.M. Brown, M. Creatore., “Opportunities of Atomic Layer Deposition for Perovskite Solar Cells,” ECS Trans., vol. 69, no. 7, pp. 15–22, 2015.
[26] A. F. Palmstrom et al., “Interfacial Effects of Tin Oxide Atomic Layer Deposition in Metal Halide Perovskite Photovoltaics,” Adv. Energy Mater., vol. 1800591, p. 1800591, 2018.
[27] D. Koushik, F. Naziris, J. Melskens, A. Nusteling, V. Zardetto, H. Schut, W.M.M. Kessels, S.W.H. Eijt, M. Creatore, “On the effect of atomic layer deposited Al2O3 on the environmental degradation of hybrid perovskite probed by positron annihilation spectroscopy,” J. Mater. Chem. C, vol. 7, no. 18, pp. 5275–5284, 2019.
[28] D. Koushik, W.J.H. Verhees, Y. Kuang, S. Veenstra, D. Zhang, M.A. Verheijen, M. Creatore, R.E.I. Schropp, “High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture,” Energy Environ. Sci., vol. 10, no. 1, pp. 91–100, 2017.
[29] Jošt, M.; Bertram, T.; Koushik, D.; Marquez, J. A.; Verheijen, M. A.; Heinemann, M. D.; Köhnen, E.; Al-Ashouri, A.; Braunger, S.; Lang, F.; Rech, B.; Unold, T.; Creatore, M.; Lauermann, I.; Kaufmann, C. A.; Schlatmann, R.; Albrecht, S. 21.6%-Efficient Monolithic Perovskite/Cu(In,Ga)Se 2 Tandem Solar Cells with Thin Conformal Hole Transport Layers for Integration on Rough Bottom Cell Surfaces. ACS Energy Lett. 2019, 4 (2), 583–590.









Very nice summary. It is really nice to see all these applications of ALD for solar cells, whether it is on a lab or on an industrial scale.
I would like to add one ALD application that has been very useful for CIGS and CZTS thin film solar cells for quite a long time now and that is the possibility to in a controlled manner change/scan the deposited layers electronic and optical properties by changing the composition of ternary compounds or by simply changing the growth conditions of binary compounds. Since CIGS and CZTS can be made with several different band gaps and band positions there is a need to adapt the neighboring layers to avoid blockage of excited electrons and holes leaving the absorber, while at the same time avoid losing Voc due to big band position mismatches. The CIGS and CZTS absorber materials typically also have fairly rough and sensitive surfaces and it has historically therefore been tough to coat them uniformly by PVD methods and achieve good solar cell performance. CBD and ALD have thus been the go to choices for developing new materials to form junctions with these absorbers. As an example using either SnOx or ZnO on top of standard composition CIGS (Eg of 1.1 eV) gives ok solar cell performance, but not great. According to theory, their conduction band levels are too low compared to the CIGS and the resulting cells will have an increased recombination and thus a lower Voc. However, by using an ALD super cycle scheme the ternary ZnSnO composition can be scanned and at the same time the bands in this case shifts. At a certain composition the bands can line up optimally with the CIGS and great solar cells can be achieved. This has recently enabled higher efficiencies to be achievable for high band gap CZTS and CGS based solar cells. In a similar manner the ternaries Zn(O,S), (Zn,Mg)O and SnGaO have also been compositionally tuned to work with CIGS. While CIGS and CZTS might see a lower interest going forward, new absorber materials are constantly being created, as in the perovskite field you mention, which might end up with new band positions that require adaptation of the existing inorganic ETL and HTL options. By using ALD in a similar way as for CIGS and CZTS before, interface engineering does not only have to become limited to trying out binary phases, but can instead include scanning different properties in between the binaries and possibly combine their strengths.